Obscure impacts demystified: Ionizing radiation
Today’s topic in our obscure impact series: ionizing radiation. We have all heard of it, but what is it really? What can we do about it? And how should we account for it in our life cycle assessments? With this series, PRé contributes to the goal of the LCA community to cover everything in their assessments, including impacts that are not directly top of mind for most people.
Warning: ionizing radiation!
Ionizing radiation is probably the impact category with the best known symbol. You might have seen it in a laboratory, hospital or post-apocalyptic blockbuster. Regardless of where you learned about it, you immediately know what the trefoil (the black circle, surrounded by three emission blocks) portrayed on a bright yellow background means: danger. But why is ionizing radiation so dangerous? And is it really that dangerous? How do we measure ionizing radiation, and how do we stop it?
What is ionizing radiation?
First, let’s take a look at how ionizing radiation is emitted. It is emitted by radioactive materials called radionuclides: elements (atoms) that have excess nuclear energy. This makes them unstable, with a chance to disintegrate into a different element. During this process, the excess energy turns into ionizing radiation – emitted as a particle or electromagnetic wave.
As the name implies, ionizing radiation can ‘ionize’ an atom or molecule. This means that it carries sufficient energy to detach electrons from atoms or molecules it encounters. A neutral atom or molecule has an equal number of positive charges (protons) and negative charges (electrons). Detachment of an electron due to ionizing radiation means that the atom or molecule is left as a positively charged ion.
Not all forms of radiation are ionizing. Non-ionizing radiation is very low in energy and therefore does not have the ability to detach electrons from other molecules. As shown in the image below, examples of non-ionizing radiation are radio waves, microwaves and visible light.
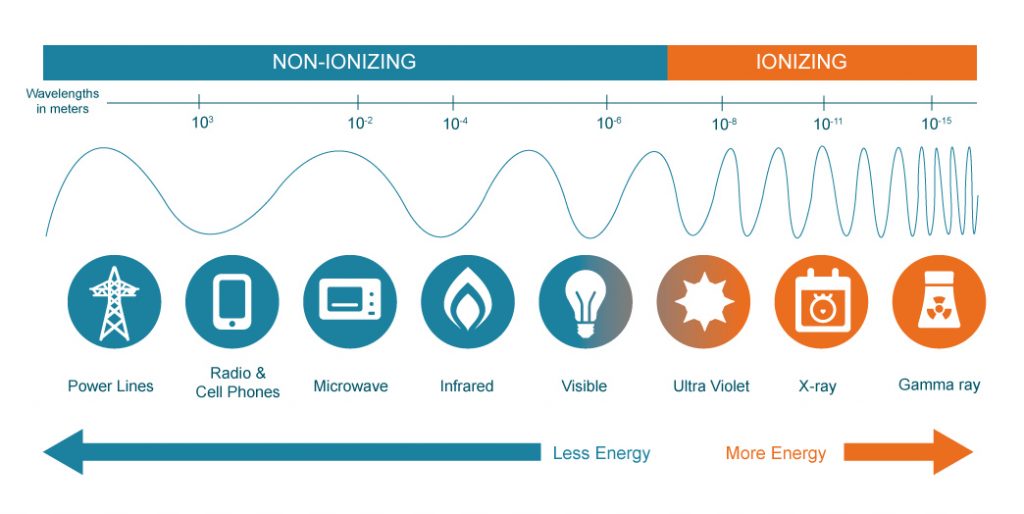
Under normal circumstances, humans are not able to detect ionizing radiation with their senses, so its presence can only be indicated and measured with specialized detection instruments such as Geiger counters. Only extremely high doses of ionizing radiation can be sensed, as a burning feeling on the skin. A detection instrument is of critical importance because significant health hazards are presented if the human body is exposed to too much ionizing radiation.
Why is it a problem?
Ionizing radiation has the potential to interact with and change molecules, and damage or even kill cells. The amount of radiation absorbed by a person’s body is called the radiation dose. Depending on this dose, serious health problems can occur because of the cell damage.
At high doses, large numbers of cells can be damaged by the radiation, possibly resulting in impaired organ functioning, skin burns or even death. A direct death because of exposure to ionizing radiation is very uncommon and only seen in extreme occasions. Examples include the atom bombs that concluded World War II or the accidents in Chernobyl and Fukushima, although the last registered only one direct death because of radiation.
More common are exposures at low doses, where fewer cells are damaged. The damaged cells can often repair themselves without any consequences. However, sometimes the affected cells are not repaired correctly, which increases the risk of long-term health problems such as cancer. Young people have increased health risks from ionizing radiation, because they have more cells that are dividing rapidly and their longer live span gives cancers more time to develop.
Ionizing radiation can also be dangerous to animals and plants. However, they mostly have much higher radio resistance, meaning that they can better withstand ionizing radiation because of cellular radioprotection mechanisms. Examples are higher levels of protective proteins, increased gene expression and altered DNA repair. Because there is no naturally occurring selection pressure that advantages organisms with better resistance to high doses of radiation, radio resistance is the result of evolutionary adaptation to different environmental extremes.
What causes ionizing radiation?
There is always a certain level of background radiation coming from natural sources. In our soils, water and the air we breathe, over 60 naturally-occurring radioactive materials can be found. This means that we inhale and ingest radionuclides on an everyday basis. This is usually not harmful, because the annual background dose is generally low. Ionizing radiation also arises from human-made sources within a large range of fields such as nuclear power generation, medical devices for diagnosis, research, manufacturing, construction.
Measuring ionizing radiation
The most common life cycle impact analysis (LCIA) methods include ionizing radiation as one of the impact categories. For instance, the ReCiPe 2016 Midpoint, the ILCD 2011 Midpoint+ and the Environmental Footprint 3.0 methods.
All methods include a wide variety of radionuclides. Some of them are well known and often associated with radiation, like uranium and plutonium. Others, like americium and ruthenium, are more unknown. For all ionizing radiative elements, both waterborne and airborne emissions are considered in LCIA methods.
How can we stop ionizing radiation?
Let’s start with the bad news: there is no way to stop it. Ionizing radiation is found everywhere. Traces of radionuclides in your granite counter top, radioactive potassium-40 in bananas and direct radiation from the sun, especially at high altitudes in planes. But the good news is, these are only low doses, and, as long you do not eat too many bananas or spend too much time in a plane, our body is able to recover from those smaller doses.
High-dose applications, like X-ray scanners and particularly nuclear energy, are much more important. Since the end of World War II, radiation has been often criticized and protested. As a consequence, high-dose applications are operated with caution and thus cause limited exposure. Nevertheless, the extraction, processing and disposal of uranium for nuclear energy production is a major source of ionizing radiation.
Therefore, it is common to see high impacts in ionizing radiation in studies where nuclear power is a major electricity source, as for example in the French electricity mix. Using less nuclear energy would be an effective way to lower the impact in ionizing radiation, however, this comes at a price. After all, nuclear energy is also a very low-carbon energy source. As often in sustainability, it is thus all about trade-offs. Luckily, we have LCA to support such decision making!
We hope you enjoyed this article! Please let us know which other LCA indicators you’d like to read about and spread the word on social media using the hashtag #ObscureImpacts.
Read about other impact categories:
Hendrik Oosterhoff
Consultant
Hendrik worked at PRé from 2020 to March 2024. Within the field of LCA, he specialized in absolute sustainability, the planetary boundaries and the application of ethical theories into assessment methodologies. As a Consultant, he collaborated on LCA and corporate footprinting and was part of the SimaPro and LCA training team.
Wouter van Kootwijk
Wouter was an intern at PRé in 2020, working on his master thesis research related to linking life cycle assessment to the UN Sustainable Development Goals (SDGs). More specifically, he investigated the use of absolute sustainability references as benchmarks for products’ environmental performance. Wouter holds a BSc in Science & Innovation Management from Utrecht University and MSc in Industrial Ecology at Leiden University and TU Delft.