Obscure impacts demystified: Stratospheric ozone depletion
The LCA community proudly strives to cover everything in their assessments: wide system boundaries, the whole lifecycle of a product and many different environmental impact categories. That means that sustainability practitioners have to be able to talk about concepts like eutrophication, photochemical ozone formation, acidification and ecotoxicity. You probably know the meaning of the words, but do you know what it means for the planet? Why is it bad? What causes it? How can we measure it? This series will give you the information you need to work with these concepts in your sustainability efforts. Today’s topic: stratospheric ozone depletion. You might think that’s a solved issue, but it isn’t.
Ozone depletion, isn’t that a thing from the ‘80s?
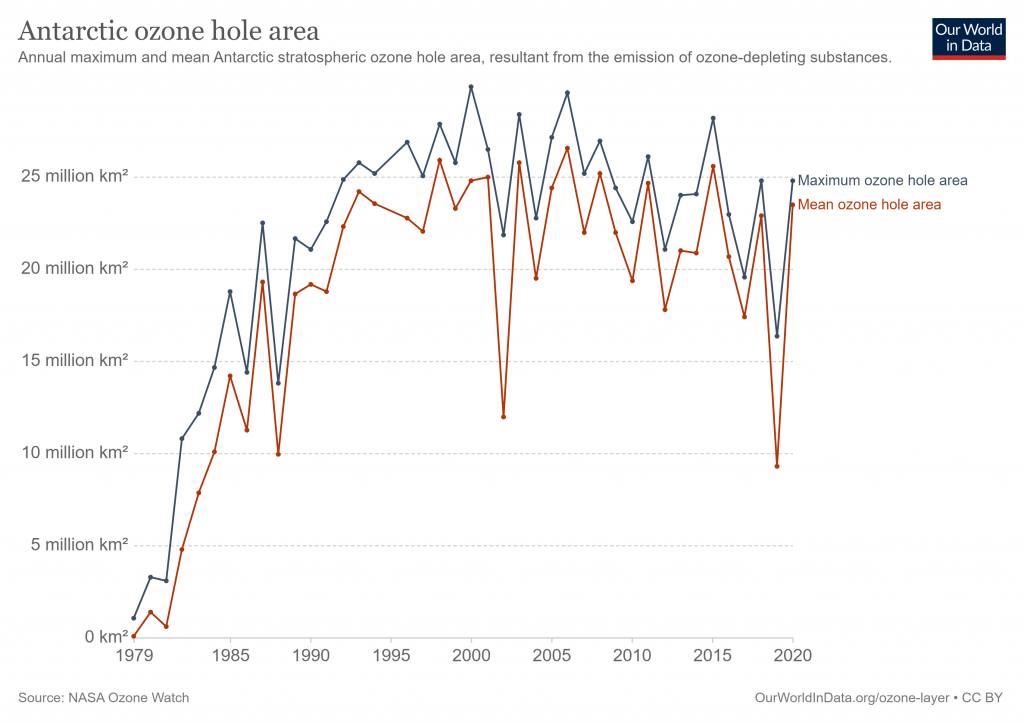
In the 1980s, a so-called “ozone hole” appeared over the Antarctic, caused by the emission of ozone-depleting substances like chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs). In a 1987 international treaty, the Montreal Protocol, it was agreed to phase out the production of some of these ozone-depleting substances. As a result, the ozone layer is slowly recovering, and last year’s ozone hole over the Antarctic was the smallest on record.
Recently, however, an ozone hole occurred over the Arctic, which is probably the biggest ever measured. This hole was mainly caused by extremely low temperatures: a polar vortex trapped unusual volumes of cold air above the Arctic. This led to the formation of high-altitude clouds, on the surface of which ozone-depleting reactions occur. Although the weather was a big causal factor, the hole over the Arctic shows stratospheric ozone depletion is still an issue.
What is stratospheric ozone depletion?
The stratosphere is the second layer in the atmosphere seen from the earth, reaching from about 10 to 50 km above sea level. Between 12 and 30 km, we find the ozone layer, with the highest ozone concentrations of the atmosphere.
Stratospheric ozone depletion is the conversion of ozone (O3) into oxygen (O2) in the stratosphere.
This happens under the influence of a number of catalysts. The most important catalysts are hydroxyl radicals (OH•), nitrous oxide radicals (NO•), chlorine radicals (Cl•) and bromine radicals (Br•). The word radical means these molecules contain an unpaired electron, which means they easily react with the ozone molecules.
As an example, this is how the chlorine radical facilitates ozone breakdown into oxygen:
Cl• + O3 → ClO + O2
ClO + O3 → Cl• + O2
After the destruction of two ozone molecules, the chlorine radical is available again to continue the destruction of other ozone molecules. This goes on until the chlorine is transported back to the troposphere (lower down in the atmosphere) or is bound in more stable molecules such as hydrogen chloride (HCl).
Why would we care?
Stratospheric ozone functions as a kind of sunscreen for the earth. It filters out the ultraviolet (UVB) of the sunlight. Loss of stratospheric ozone can have a multitude of effects, since increased UVB radiation can:
- Damage DNA and cause skin cancer and cataracts (a clouding of the eye’s lens) in humans.
- Reduce plant growth by affecting the physiological processes and development.
- Reduce phytoplankton production. Phytoplankton is the foundation of many aquatic food webs. Since UVB radiation can also damage some developmental stages of small marine organisms, increased UVB radiation has implications for the whole marine food chain.
- Affect biogeochemical cycles which may alter sources and sinks of greenhouse gases and chemically important gases.
- Affect materials such as polymers leading to a reduced lifetime of certain outdoor products.
Quick guide: biogeochemical cycles
Biogeochemical cycles are pathways via which a chemical substance moves through different compartments of the earth. Soil, water, atmosphere and living organisms are examples of such compartments. These different compartments are linked through feedback loops, an increase of the chemical in one compartment could have a positive or negative effect on that chemical in another compartment.
What is causing it?
The catalysts that break down ozone in the atmosphere come from both natural and man-made sources. Man-made catalysts are also called ozone-depleting substances, such as CFCs and HCFCs used in refrigerators, air conditioning, and heat pump systems; halons used for fire suppression; and methyl bromide used as pest control.
Most of these ozone-depleting substances were phased out after the Montreal Protocol. However, it will take some time before the ozone-depleting substances disappear completely from the atmosphere. The Montreal Protocol also doesn’t include the ozone-depleting substance nitrous oxide (N2O) which leads to the formation of the nitrous oxide radical.
Ozone depletion in LCA
To determine if and how much a product or service contributes to stratospheric ozone depletion, sustainability professionals can include these ozone-depleting substances in LCA. Most of the life cycle impact analysis (LCIA) methods include ozone-depleting substances.
However, not all LCIA methods include N2O as an ozone-depleting substance, since its ozone-depleting potential (ODP) over a longer period of time is more uncertain. ReCiPe 2016 does include N2O but notes that the ODP’s for it should be considered preliminary.
Stratospheric ozone depletion: in conclusion
Although the Montreal Protocol successfully phased out most of the ozone-depleting substances and the ozone layer is recovering, stratospheric ozone depletion is still an issue. Since it has several severe adverse effects on human health, plant growth, marine food chains and biogeochemical cycles, it is important to remove the source of stratospheric ozone depletion.
Because current ozone-depleting substances will be active for a long time yet, it is extra important to reduce the amount that goes up into the atmosphere. Incorporating stratospheric ozone depletion in LCA and acting on reducing it is key.
We hope you enjoyed this article! Please let us know which other LCA indicators you’d like to read about and spread the word on social media using the hashtag #ObscureImpacts. If you want to receive quarterly articles about sustainability and life cycle thinking in your inbox, subscribe to our newsletter in the form below.
Read about other impact categories:
Laura Schumacher
Expert
I believe the current human influence on earth systems is not sustainable. To lower the impact of humans on earth systems, this impact first needs to be understood. By understanding the environmental impact of daily practices, improvement options can be identified. Sustainability metrics provide this insight into the environmental impact. Working in sustainability metrics combines my passion for modelling and understanding the influence of humans on earth systems with my drive to lower the environmental impact of daily practices.